Impact of HCMV-encoded FcγR antagonists on innate immune cell function
Human cytomegalovirus (HCMV) infection persists for life and is characterized by alternating phases of productive and latent infection. HCMV co-evolution with the human host has produced a very large array of highly specialized immune evasive viral genes and unique abilities of the virus to reprogramme complex host immune functions (1). Such a singularity of HCMV infection is the induction of so-called ‘adaptive’ or ‘memory-like’ NK cells which are characterized by a stable redistribution of their NK cell receptor (NKR) repertoire, profound alterations in signaling molecules, transcription factors and epigenetic changes compared with ‘canonical’ NK cells (2). Remarkably, ‘adaptive’ NK cells are found in highly variable numbers in HCMV-infected individuals, indicating that additional factors drive their expansion in vivo. The hallmark of this process is the expansion of mature NK cell subsets displaying high levels of the CD94/NKG2C receptor (NKG2Cbright) associated with distinctive phenotypic and functional features like down-regulation of the FcεR-γ chain (3,4).
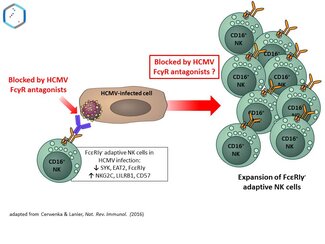
Fig. 1: Manipulation of FcγR-mediated effector functions and adaptive NK cell expansion by HCVM-encoded antagonists
Importantly, adaptive NK cells are particularly proficient to execute antibody-dependent cell-mediated cytotoxicity (ADCC) via FcγRIII/CD16 (4), one of the strongest activatory receptors expressed by a large subset of NK cells. Conversely, HCMV employs a variety of counter-strategies to evade NK cell-mediated immune control. Our laboratory has identified three HCMV-encoded Fcγ binding glycoproteins co-expressed during infection, i.e. the HCMV surface resident molecules gp34 (RL11), gp68 (UL119-118) and gp95 (RL12) (5,6). We demonstrated that gp34, gp68 and gp95 invariably block IgG-mediated activation of host FcγRs like ADCC (antibody-dependent cellular cytotoxicity) of NK cells mediated by FcγRIII/CD16 (7).
Notably, expansion of ‘adaptive” NK cells can be recapitulated in vitro (8,9). In this project we hypothesize i) that HCMV-specific IgG contributes essentially to the expansion of adaptive NK cells via FcγRIII/CD16 and ii) that HCMV FcγR antagonists could counteract this crucial effect. Moreover, our project aims for an elucidation of distinct molecular mechanisms by which HCMV-encoded FcγR antagonists execute their obstructive function and achieve synergistic effects when acting in concert during infection.
References
- Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 160:37-47 (2015).
- Lee, J., et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 42:431-42 (2015).
- Guma, M., et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 104: 3664-71 (2004).
- Zhang, T., et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol. 190:1402-6 (2013).
- Atalay R, Zimmermann A, Wagner M, Borst, E, Benz C, Messerle M, Hengel H. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcγ-receptor homologs. J. Virol. 76:8596–608 (2002).
- Corrales-Aguilar E, Hoffmann K, Hengel H. CMV-encoded Fcγ receptors: modulators at the interface of innate and adaptive immunity. Semin. Immunopathol. 36:627-40 (2014).
- Corrales-Aguilar E, Trilling M, Hunold K, Fiedler M, Le VTK, Reinhard H, Ehrhardt K, Mercé-Maldonado E, Alijev E, Zimmermann A, Johnson DC, Hengel H. Human cytomegalovirus Fcγ binding proteins gp34 and gp68 antagonize Fcγ receptors I, II and III. PLoS Pathog. 10(5):e1004131(2014).
- Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 107:3624-31 (2006).
- Rölle A, Pollmann J, Ewen E-M, Halenius A, Hengel H, Cerwenka A. CD14+cells, IL-12 and HLA-E drive NKG2C+NK cell expansion in HCMV infection. J. Clin. Invest. 124:5305–16 (2014).
Funding:
DFG HE 2526/9-1 - Research Unit “Advanced Concepts in Cellular Immune Control of Cytomegalovirus (FOR 2830)” http://www.virologie.uni-wuerzburg.de/forschungsverbuende/for2830/home/
InfectERA Consortium TANKACY TArgeting Natural Killer cells Against Cytomegalovirus http://www.infect-era.eu/3th-call-2015
NIH consortium "Immunologic and Virologic Determinants of Congenital Cytomegalovirus Transmission and Disease in Rhesus Monkeys”, Grant Number: 1P01AI129859-01A1

Head:
Prof. Dr. med. Hartmut Hengel
hartmut.hengel@uniklinik-freiburg.de
| Secretary | Administration | Information desk |
|---|---|---|
Kristina Gendrisch Telefon: 0761 270-83480 Telefax: 0761 270-83479 | Gudrun Simpson Telefon: 0761 270-83711 Telefax: 0761 270-83703 | Jutta Schneeberger Telefon: 0761 270-83700 Telefax: 0761 270-83703 |