CyTOF-Facility
Highly multiplexed profiling of single cells and tissues are emerging as essential technologies to decipher complex biological phenotypes in health and disease. Mass cytometry (CyTOF – Cytometry by Time of Flight) and imaging mass cytometry (IMC) allow rapid and efficient multiplexing of isotope-labelled detection reagents for analysis of single-cell solutions or tissue slides. These technologies are revolutionizing our understanding of systemic immune responses.
Mass Cytometry: Comprehensively profile cell phenotypes and functional states using CyTOF technology
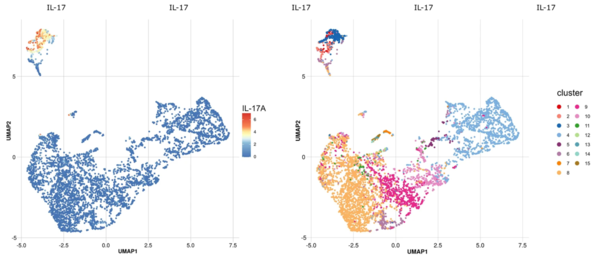
Mass cytometry is a novel platform for high-dimensional phenotypic and functional analysis of single-cells. Cellular targets can be labeled with more than 40 metal-tagged antibodies. These are detected and quantified by time of flight (TOF) performed on the Helios CyTOF system with minimal overlap between channels. Data is transformed into FCS files which are compatible with most of Flow Cytometry software but is also ideal for bioinformatics approaches.
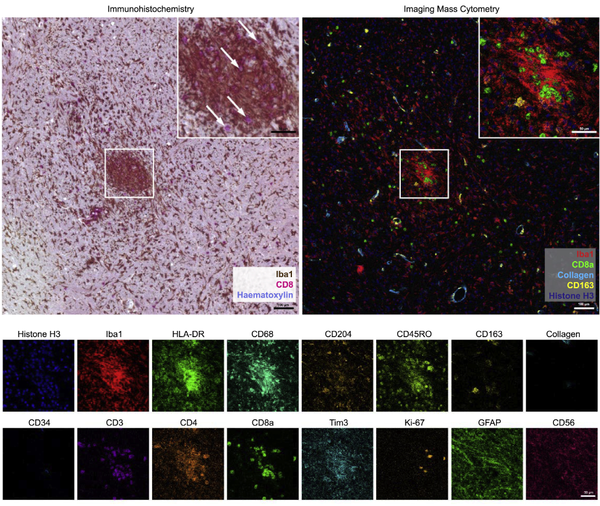
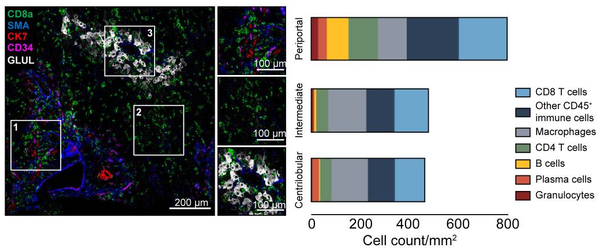
Imaging Mass Cytometry: Deeply interrogate cellular phenotypes in the spatial context of the tissue environment; Visualize pathology and disease with high-multiplex imaging
Given the importance of spatial resolution to studies of cellular interplay, the Imaging Mass Cytometry workflow enables deep profiling of standard FFPE or frozen tissue sections and of fixed cells deposited on glass microscope slides using the Hyperion Imaging System. Computerized analysis of the generated images involves the segmentation of intact cells, nuclei, or other objects of interest, followed by quantification of the expression level of each of the analyzed markers. The high‐dimensional single‐cell data thus generated can then be addressed using tools similar to those used for high parameter flow cytometry data.
Suspension CyTOF
- Currently up to 50 markers measured simultaneously
- Additional channels for cell identification and viability
- Sample barcoding to multiplex up to 20 samples in a single tube
- Minimal signal overlap, no compensation necessary
- Low biological background contaminations
- Beads added to samples allows for data normalization
Imaging mass cytometry
- Mass-based targeted Imaging
- Simulatenous measurement of 40+ targets
- 1µm resolution
- FFPE and fresh frozen compatible
We support users in project planning, experimental design, panel design, sample preparation, optimization, data acquisition and basic data analysis.
The Freiburg Mass Cytometry Unit utilizes a 3rd generation CyTOF Helios and Hyperion platform. We provide core functionality for collaborative projects, ranging from consultation, reagent conjugation, standardized staining work flows, training and data acquisition to guidance with bioinformatics analysis. Frequently, standardized core panels can be rapidly adapted to individual research needs.
External users are welcome – if interested please contact us for further information.
We are supported by the German Research Foundation (DFG) and the Faculty of Medicine, University of Freiburg.
Nat Commun 13, 3688 (2022)
High-dimensional profiling reveals Tc17 cell enrichment in active Crohn’s disease and identifies a potentially targetable signature.
Globig AM, Hipp AV, Otto-Mora P, Heeg M, Mayer LS, Ehl S, Schwacha H, Bewtra M, Tomov V, Thimme R, Hasselblatt P, Bengsch B.
Nat Commun 13, 3688 (2022). https://doi.org/10.1038/s41467-022-31229-z
Immunity. 2021 Jul 13;54(7):1594-1610.e11
Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions.
Schwabenland M, Salié H, Tanevski J, Killmer S, Lago MS, Schlaak AE, Mayer L, Matschke J, Püschel K, Fitzek A, Ondruschka B, Mei HE, Boettler T, Neumann-Haefelin C, Hofmann M, Breithaupt A, Genc N, Stadelmann C, Saez-Rodriguez J, Bronsert P, Knobeloch KP, Blank T, Thimme R, Glatzel M, Prinz M, Bengsch B.
Immunity. 2021 Jul 13;54(7):1594-1610.e11. https://doi.org/10.1016/j.immuni.2021.06.002
J Hepatol. 2022 Aug;77(2):397-409
T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma.
Barsch M, Salié H, Schlaak AE, Zhang Z, Hess M, Mayer LS, Tauber C, Otto-Mora P, Ohtani T, Nilsson T, Wischer L, Winkler F, Manne S, Rech A, Schmitt-Graeff A, Bronsert P, Hofmann M, Neumann-Haefelin C, Boettler T, Fichtner-Feigl S, van Boemmel F, Berg T, Rimassa L, Di Tommaso L, Saeed A, D'Alessio A, Pinato DJ, Bettinger D, Binder H, John Wherry E, Schultheiss M, Thimme R, Bengsch B.
J Hepatol. 2022 Aug;77(2):397-409. https://doi: 10.1016/j.jhep.2022.02.032
J Hepatol . 2022 Sep;77(3):653-659.
SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis.
Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B.
J Hepatol. 2022 Sep;77(3):653-659. https://doi.org/10.1016/j.jhep.2022.03.040
CyTOF Technical Operator (m/f/d)
We are seeking a highly motivated individual with a passion for exciting state-of-the art technology to work with a team of translational immunologists to lead the mass cytometry and imaging facility in the Bengsch lab as part of the Translational EXperimental IMmunology labs at the University of Freiburg, Germany.
Key Responsibilities:
- operation, maintenance and use of the CyTOF Helios and the HyperionImaging System
- build and optimize mass cytometry and imaging cytometry antibody panels
- conjugation and QC of mass cytometry reagents
- consult with users regarding potential mass cytometry projects which may include guiding experimental set-up and procedure optimization, panel design, and data analysis consultation
- basic data quality assessment
- perform and analyze mass cytometry and imaging experiments
- act as a liaison between users and applications specialist
Key Requirements:
- PhD degree in immunology, biological sciences, biochemistry or related field (other technical and academic (diploma/Master’s) degrees will be considered based on research experience)
- demonstrated skill with flow or mass cytometry (previous experience with mass cytometry is a plus) (sample preparation, instrument optimization and data analysis)
- experience with Immunofluorescence and/or Immunohistochemistry techniques or Imaging Mass cytometry (slide preparation staining optimization visualization and data analysis).
- interest in bioinformatics, experience with basic flow cytometry software and R preferred
- ability to identify and troubleshoot critical issues
- detail-oriented
- excellent communication and organizational skills
What we offer:
- exciting translational research
- work in an interdisciplinary team
- being part of the German Mass Cytometry Network
- hospital-standard social benefits, e.g., UKF job ticket
- employment according to TV-L according to academic qualification
- Freiburg is an attractive University city next to the Black Forest in Germany, that is also highly international, "green" and also considered a top destination close to France and Switzerland surrounded by a beautiful landscape
Please direct your informative applications with CV and letter of motivation to
masscytometry@uniklinik-freiburg.de or contact Bertram Bengsch (PI) directly.