Quantitative PCR
qPCRLighthouse has two Roche LightCycler 480 (LC 480) machines for quantitative PCR analysis. Each LC 480 is a plate-based machine with both 384-well and 96-well blocks. The thermal blocks are fast-cycling, with typical run times slightly under one hour. The range of reaction volumes is 20 - 100 microliters for the 96-well and 5 - 20 microliters for the 384-well block.
The LC 480 has a number of possible excitation/emission wavelengths to choose from, making multiplexing much easier. The table below lists the filters present in each machine. Excitation/emission filters can be set independently.
| Emission Wavelengths (nm) LC1 (Obi Wan) | Emission Wavelengths (nm) LC2 (Luke Skywalker) | Compatible Dyes |
500/20 | 488/20 | LightCycler (LC) Cyan 500 |
533/20 | 510/20 | SYBR Green, FAM, TET |
568/20 | 580/20 | HEX, VIC, TAMRA, CY3 |
610/20 | 610/20 | LC RED 610 |
640/20 | 640/20 | LC RED 640 |
| 670/20 | 660/95 | CY5 |
When multiplexing we suggest taking advantage of the broad range of the LC480 by using dyes whose emission spectra lie far apart. This limits the amount of spectral overlap between the probes, so that color compensation is no longer necessary. Before ordering your probes we recommend that you talk to us first. See the bottom of the page for some useful PDF files about quantitative PCR.
Hydrolysis (TaqMan) Probes: How do they work?
Real-time quantitative PCR analysis often involves the use of one or more hydrolysis probes. The principle behind their use is explained in the following:
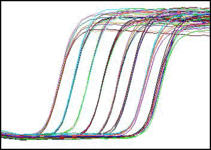
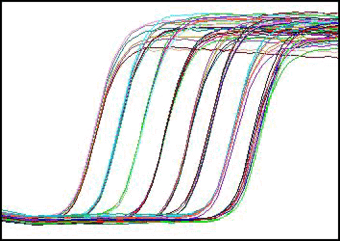
This figure shows the amplification of samples from a series of 10-fold dilutions. Reporter signal is plotted against time.
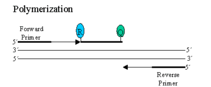
Two fluorescent dyes, a reporter (R) and a quencher (Q), are attached to the hydrolysis probe. The 3' end of the probe is blocked, so it is not extended during the PCR reaction.
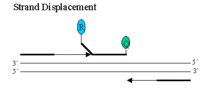
When both dyes are attached to the probe, reporter dye emission is quenched due to fluorescence energy transfer (FRET) from the reporter dye to the quencher dye. During each extension cycle, the probe is displaced at the 5' end by the DNA polymerase.
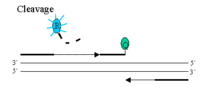
Taq DNA polymerase then cleaves the reporter dye from the probe via its 5'-3' exonuclease. This exonuclease functions best at 60o, which is why the extension step is normally at that temperature.
Once separated from the quencher, the reporter dye emits its characteristic fluorescence which can then be measured on the LC 480. The amount of fluorescence measured is proportional to the amount of PCR product made.
The design of hydrolysis probes and primers is critical to the success of the experiment. We have a copy of the Beacon Designer program to help you out with probe and primer design.
SYBR Green Chemistry:
It is also possible to use the DNA binding dye SYBR Green for QPCR. This dye fluoresces only when bound to double-stranded DNA, and offers an alternative way to label PCR products, without having to construct a new hydrolysis probe for each assay. Because the dye binds all double stranded nucleic acids present, including primer dimers, it is necessary to perform a melting curve analysis at the end of each run.
Measurement of Fluorescence:
During the cycling process, fluorescence emission is measured every cycle at the appropriate wavelength(s) within each well. After the run, the results are analyzed and can be examined in a number of ways, one of which is the amplification plot shown below:
Some Useful Documents about Quantitative PCR:(Also be sure to check out the Quantitative PCR Links of Interest for some useful external websites.) | |
| Article discussing the MIQE guidelines (Minimum Information for Publication of Quantitative Real-Time PCR Experiments). The place to start when first planning your experiment. | |
| Very informative introduction to TaqMan reactions, including tips for primer design and reaction optimization (in German). (725kB) | |
| ABI's User Bulletin Number 2: Gives lots of general information about relative quantitation and tips to help you get started. (630kB) | |
| ABI's User Bulletin Number 5: Information about multiplexing reactions. (230kB) | |
| Information from Roche about relative quantification. (837kB) | |
| Help in setting baselines and thresholds when using the fit points method (312kB) | |
| Useful tips from Roche offering help in limiting primer dimers. (435kB) | |
| Instructions on reconstituting and diluting primers and probes. (102kB) | |
For more information about using the machine or for advice in designing possible probes/primers, please contact the Lighthouse Core Facility.