Molecular Mechanisms of Immune Dysregulation
Dr. Laura Gámez-DíazWe investigate the molecular mechanisms linking autophagy, cytoskeleton and cell metabolism to immune dysregulation syndromes, including inborn errors of immunity (IEI), chronic inflammation, autoimmunity, and cancer. Our long-term goal is to identify novel targets for the diagnosis and treatment of these immune disorders.
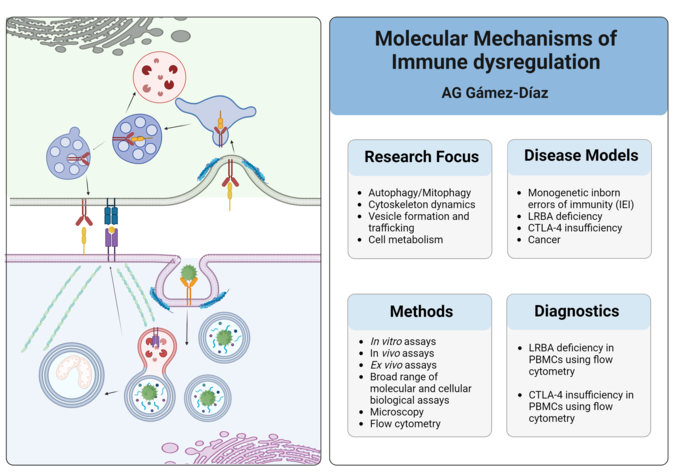
Autophagy - the major cellular waste disposal and recycling system of the cells - ensures the proper functioning of several immune processes, including pathogen recognition, antigen processing and presentation, lymphocyte development and effector function, cell metabolism and inflammatory regulation. Dysfunctional autophagy has been implicated in numerous autoimmune diseases, including inflammatory bowel disease.
In addition, essential immune response processes such as cell migration, adhesion, division, vesicle trafficking, and cellular transduction rely on a finely tuned actin cytoskeleton dynamics, involving the assembly and disassembly of actin filaments. Human monogenetic immune disorders caused by deleterious mutations in actin regulatory genes are known as actinopathies.
Finally, activation, differentiation and function of immune cells are highly dependent on energy supply and metabolic conversion. Thus, immune metabolism provides new avenues for treatment of inflammatory diseases including autoimmunity and cancer.

From left to right: Jorrell Rush-Kittle (PhD student), Anna Lang (M.Sc. student), Dr. Laura Gaméz-Díaz, Elena Sindram (PhD student), Rebecca Marsiske (B.Sc. student) and Sonja Breuning (B.Sc. student).
Ten most important publications:
- FIP200 Phosphorylation Regulates Late Steps in Mitophagy. Eickhorst C, Babic R, Rush-Kittle J, Lucya L, Lami Imam F, Sánchez-Martín P, Hollenstein D, Michaelis J, Münch C, Meisinger C, Slade D, Gámez-Díaz L*, Kraft C*. (2024). J Mol Biol. doi: 10.1016/j.jmb.2024.168631 (*corresponding)
- LRBA balances antigen presentation and T-cell responses by facilitating autophagy through the binding to PIK3R4 and FYCO1. Sindram E, Deau MC, Ligeon LA, Sanchez-Martin P, Nestel S, Jung S, Ruf S, Mishra P, Proietti M, Günther S, Thedieck K, Roussa E, Rambold A, Münz C, Kraft C, Grimbacher B and Gámez-Díaz L (2024). bioRxiv. doi.org/10.1101/2022.10.17.512524
- Functional Relevance of CTLA4 Variants: An Upgraded Approach to Asses CTLA4-Dependent Transendocytosis by Flow Cytometry. Rojas-Restrepo J, Sindram E, Zenke S, Haberstroh H, Mitsuiki N, Gabrysch AM, Huebscher K, Posadas-Cantera S, Krausz M, Kobbe R, Rohr J, Grimbacher B* and Gámez-Díaz L* (2023) J. Clin. Immunol. doi: 10.1007/s10875-023-01582-9 (*corresponding)
- ARPC5 deficiency leads to severe early-onset systemic inflammation and mortality. Sindram E, Caballero-Oteyza A, Kogata N, Allizadeh Z, Gámez-Díaz L, Reza-Fazlollhi M, Peng X, Grimbacher B, Way M, Proietti M. (2023) Dis Mod Mech. , doi: 10.1242/dmm.050145
- Do common infections trigger disease-onset or -severity in CTLA-4 insufficiency? Krausz M, Mitsuiki N, Falcone V, Komp J, Posadas-Cantera S, Lorenz HM, Litzman J, Wolff D, Kanariou M, Heinkele A, Speckmann C, Häcker G, Hengel H, Gámez-Díaz L, Grimbacher B. (2022) Front Immunol. doi: 10.3389/fimmu.2022.1011646
- Refractory autoimmune gastritis responsive to abatacept in LRBA deficiency. Boz V, Valencic E, Girardelli M, Pin A, Gámez-Díaz L, Tommasini A, Lega S, Bramuzzo M. (2021). Front Immunol. doi: 10.3389/fimmu.2021.619246
- Genetic Analysis of a Cohort of 275 Patients with Hyper-IgE Syndromes and/or Chronic Mucocutaneous Candidiasis. Frede N, Rojas-Restrepo J, Caballero Garcia de Oteyza A, Buchta M, Hübscher K, Gámez-Díaz L, Proietti M, Saghafi S, Chavoshzadeh Z, Soler-Palacin P, Galal N, Adeli M, Aldave-Becerra JC, Al-Ddafari MS, Ardenyz Ö, Atkinson TP, Kut FB, Çelmeli F, Rees H, Kilic SS, Kirovski I, Klein C, Kobbe R, Korganow AS, Lilic D, Lunt P, Makwana N, Metin A, Özgür TT, Karakas AA, Seneviratne S, Sherkat R, Sousa AB, Unal E, Patiroglu T, Wahn V, von Bernuth H, Whiteford M, Doffinger R, Jouhadi Z, Grimbacher B. (2021). J Clin Immunol. doi: 10.1007/s10875-021-01086-4
- Rapid flow cytometry-based test for the diagnosis of LRBA deficiency. Gámez-Díaz L, Sigmund E*, Reiser V*, Vach W, Jung S, Grimbacher B. (2018). Front Immunol. doi: 10.3389/fimmu.2018.00720
- Immunological phenotype of the murine Lrba knockout. Gámez-Díaz L, Neumann J, Jäger F, Proietti M, Felber F, Soulas-Sprauel P, Perruzza L, Grassi F, Kögl T, Aichele P, Kilimann M, Grimbacher B* and Jung S (2017). Immunol Cell Biol. doi: 10.1038/icb.2017.52
- The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. Gámez-Díaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, Morio T, Worth AJ, Blessing J, Van de Veerdonk F, Feuchtinger T, Kanariou M, Schmitt-Graeff A, Jung S, Seneviratne S, Burns S, Belohradsky BH, Rezaei N, Bakhtiar S, Speckmann C, Jordan M, Grimbacher B. (2016). J Allergy Clin Immunol. doi: 10.1016/j.jaci.2015.09.025.
All publications are available on orcid.org/0000-0002-6800-9736

Center for Chronic Immunodeficiency
at Center for Translational Cell Research
Breisacher Str. 115
79106 Freiburg
Germany

