LRBA deficiency
Dr. Laura Gámez-Díaz
LRBA deficiency was firstly described by the group of Prof. Grimbacher in children with antibody deficiency, harboring biallelic mutations in LRBA that abolish its protein expression (Lopez-Herrera et al, Am J Hum Genet). Clinically, LRBA deficiency presents with a broad clinical phenotype including mostly autoimmune cytopenia, enteropathy, recurrent infections, and hypogammaglobulinemia, accompanied by reduced expression of CTLA-4 as well as diminished numbers of regulatory T cells (Tregs), switched memory B cells, and plasmablasts (Gámez-Díaz et al., J Allergy Clin Immunol. 2016). Interestingly, LRBA deficiency mouse models do not mirror the human LRBA deficiency, as Lrba null mice do not present any clinical signs of disease and do not develop overt autoimmunity despite low levels of CTLA-4, reduced frequency of IL-10-producing B cells, and increased numbers of T follicular helper cells (Gámez-Díaz L et al., Immunol Cell Biol. 2017). In addition, Lrba null mice have a normal B- and T lymphocyte development and normal humoral immune responses against T-dependent as well as T-independent antigens (Gámez-Díaz L et al., Immunol Cell Biol. 2017).
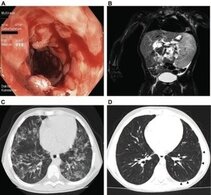
A) Colonoscopy in a LRBA-deficient patient (P105) revealed severe circular inflammation in the rectum, numerous ulcerations, and mucosal edema and erythema.
B) Magnetic resonance image from a LRBA-deficient patient (P109) showing marked splenomegaly.
C) High- resolution chest computed tomographic scan from patient 105 showing atypical, partly conflating patchy alveolar consolidations in both lungs.
D) Marked regression of alveolar consolidation 2 years after therapy and slight bilateral ground-glass infiltrates in patient 105.
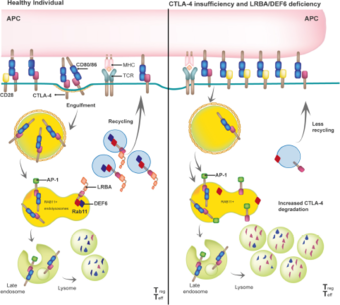
Biologically, LRBA plays an essential role in the trafficking of CTLA-4 in Tregs, thereby controlling T-cell mediated immune responses (Lo et al., Science, 2015). However, some preliminary results suggest that LRBA acts as a scaffold protein necessary for other endosomal trafficking routes in a cell-specific and cell- metabolic state manner (own unpublished data) (Figure 2).
LRBA binds with high affinity to the tail of CTLA-4, which is expressed constitutively in regulatory T cells (Tregs) or upon activation in conventional T cells. The LRBA- CTLA-4 interaction is required to guide CTLA-4 containing vesicles to the cell surface in order to capture and engulf the co-stimulatory molecules CD80 and CD86 from the antigen presenting cells (APC), thereby effectively controlling T-cell activation (left panel). In the absence of LRBA, the adaptor protein AP-1 binds to CTLA-4 leading to its lysosomal degradation, resulting in a reduced overall CTLA 4 (as observed in CTLA4 insufficiency patients) that promotes sustained and prolonged T cell activation. Gamez-Diaz, Front. Immuno. 2021.
Selected Publications
- L Gámez-Díaz and M Seidel. Different apples, same tree: Biological and clinical insights into CTLA-4 insufficiency, LRBA and DEF6 deficiencies. Front. Immunol. 2021. Review.
- Victoria Katharina Tesch, Hassan Abolhassani, Bella Shadur, Joachim Zobel, Yuliya Mareika, Svetlana Sharapova, Elif Karakoc-Aydiner, Jaques G. Rivière, Marina Garcia-Prat1, Nicolette Moes, Filomeen Haerynck, Luis I. Gonzales- Granado, Juan Luis Santos Pérez, Anna Mukhina, Asghar Aghamohammadi, Lennart Hammarström, Figen Dogu, Aydan İ İkincioğulları, Sevgi Bal, Safa Baris, Sara Sebnem Kilic, Neslihan Edeer Karaca, Necil Kutukculer, Hermann Girschick, Antonios Kolios, Sevgi Keles, Polina Stepensky, Austen Worth, Joris van Montfrans, Anke Peters, Isabelle Meyts, Sevgi Keles, Antonio Marzollo, Npadem Nurcicek, Zahra Chavoshzadeh, Magdalena Avbelj, Shahrzad Bakhtiar, Benoit Florkin, Marie Meeths, Laura Gamez, Bodo Grimbacher, Mikko Seppänen, Arjan Lankaster, Andrew Gennery, and Markus G Seidel. Long-term outcome of LRBA deficiency in 76 patients after various treatment modalities evaluated by the immune deficiency and dysregulation activity (IDDA) score. J Allergy Clin Immunol. 2020 May; 145 (5): 1452-1463.
- De Bruyne M, Bogaert DJ, Venken K, Van den Bossche L, Bonroy C, Roels L, Tavernier SJ, van de Vijver E, Driessen A, van Gijn M, Gámez-Díaz L, Elewaut D, Grimbacher B, Haernck F, Moes N, Dullaers M. A novel LPS-responsive beige-like anchor protein (LRBA) mutation presents with normal cytotoxic T-lymphocyte-associated protein (CTLA-4) and overactive TH17 immunity. J Allergy Clin Immunol. 2018 Dec;142 (6):1968-1971.
- Jung S, Gámez-Díaz L, Proietti M, Grimbacher B. "Immune TOR-opathies", a Novel Disease Entity in Clinical Immunology. Frontiers Immunology. 2018 May 9; 9:966. Review.
- Gámez-Díaz L, Sigmund E*, Reiser V*, Vach W, Jung S, Grimbacher B. Rapid flow cytometry- based test for the diagnosis of LRBA deficiency. Frontiers Immunology, 2018 Apr 23; 9:720.
- Gámez-Díaz L, Neumann J, Jäger F, Proietti M, Felber F, Soulas-Sprauel P, Perruzza L, Grassi F, Kögl T, Aichele P, Kilimann M, Grimbacher B* and Jung S*. Immunological phenotype of the murine Lrba knockout. Immunol Cell Biol. 2017 Oct;95(9):789-802.
- Gámez-Díaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, Morio T, Worth AJ, Blessing J, Van de Veerdonk F, Feuchtinger T, Kanariou M, Schmitt-Graeff A, Jung S, Seneviratne S, Burns S, Belohradsky BH, Rezaei N, Bakhtiar S, Speckmann C, Jordan M, Grimbacher B. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016 Jan;137(1):223-30.
- Seidel MG, Hirschmugl T, Gámez-Díaz L, Schwinger W, Serwas N, Deutschmann A, Gorkiewicz G, Zenz W, Windpassinger C, Grimbacher B, Urban C, Boztug K. Long-term remission after allogeneic hematopoietic stem cell transplantation in LPS-responsive beige-like anchor (LRBA) deficiency. J Allergy Clin Immunol. 2015 May;135(5):1384-90.
Funding agencies
Our work on LRBA deficiency has been funded by the:
- Bundesministerium für Bildung und Forschung (BMBF)
- Deutsche Forschungsgemeinschaft (DFG)
- Jeffrey Modell Foundation
- Fritz Thyssen Stiftung
- SFB1380- Young Investigator Grant
Team
| Sub group leader | ||
| Laura Gámez-Diaz | laura.gamez@uniklinik-freiburg.de | 270-77742 |
Scientists | ||
| Marie-Celine Deau | marie-celine.deau@uniklinik-freiburg.de | 270-77732 |
Students | ||
| Elena Sindram | elena.sindram@uniklinik-freiburg.de | 270-77725 |
| Türkan Çakar | 270-77754 | |
| Jorrell Rush-Kittle | rush-kittle.jorrell@uniklinik-freiburg.de | 270-77754 |
| Vanessa Zeidler | vanessa.zeidler@uniklinik-freiburg.de | 270-77754 |
Contact
MEDICAL CENTER - UNIVERSITY OF FREIBURG
Center for Chronic Immunodeficiency
Center for Translational Cell Research (ZTZ)
Breisacher Str. 115
79106 Freiburg, Germany