4D Imaging / Cardiac NanoDynamics
We use experimental approaches to investigate cardiac ultrastructure on a nano-to-micro scale. Through our research we attempt to elucidate how mechanics (i.e. active such as cell deformation, and passive such as changes in stiffness and ability to bear load) affects electrophysiology and contractile activity of the heart, and vice versa. We are also interested in novel ways the many different cell types present in the heart communicate with one other.
Main research areas are:
1. Organelle deformation during cardiac cycle – structure and functional consequences
The heart is a mechanically-active and -responsive organ, continuously adapting its passive and active mechanical properties in response to changes in demand (for example during exercise, pregnancy, early ‘adaptive’ stages of heart failure) on a beat-by-beat basis as well as with long-lasting remodelling.
One of our main research foci is how intracellular structures inside cardiac muscle cells deform, and how this deformation affects the function of the heart. We specifically investigate the transverse(T)-tubules (TT), sarcoplasmic reticulum, caveolae, mitochondria, and cytoskeleton. We probe the structure (using advanced imaging methods: confocal, multiphoton, and electron microscopy) and function (contractility, ion diffusion) in cells subjected to mechanical manipulations, such as electrical stimulation and stretch of both single cells and multicellular preparations.
A: 3D TT network in a ventricular cardiomyocyte, reconstruction based on confocal microscopy. B: Electron tomography reconstruction, TT (green), sarcoplasmic reticulum (yellow), mitochondria (blue), microtubule (red). C: TT are deformed (squeezed) during stretch and contraction, leading to faster TT content mixing (D).
2. Myocyte-nonmyocyte cross talk in the heart
Many scars – such as in skin – are a-cellular and predominantly composed of fibrillar collagen. In the heart however, fibrotic tissue is very much ‘alive’, with the ubiquitous network of the extracellular matrix (ECM) providing a scaffold for structural and mechanical integration of cells embedded within it. We work to identify novel factors that can influence the bi-directional cross-talk between different cell types. We use a combination of live cell manipulations (genetic, environmental, pharmacological), imaging, and biochemical methods. We use human material provided by the CardioVascular BioBank, to investigate human cardiac diseases such as heart failure and congenital heart defects.
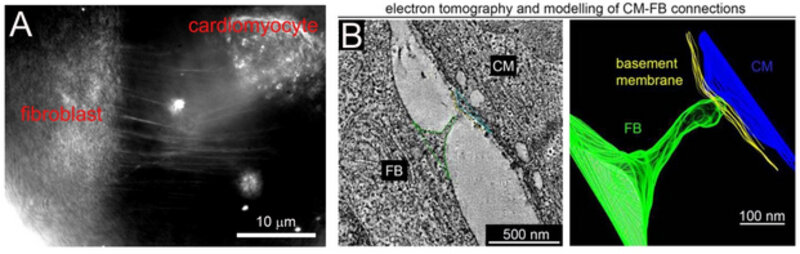
A: Membrane protrusions between a cardiomyocyte and a fibroblast, visualised in vitro using rotating coherent scattering microscopy. B: Electron tomography and reconstruction of cardiomyocyte (CM) and a fibroblast (FB) in infarct border zone.
- Mechanical deformation of myocyte ultrastructure – structure and function
- Quantitative T-tubular network analysis
- Development of novel live fluorescent ion indicators
- Fibrotic remodelling in Tetralogy of Fallot
- In vitro fibrosis model
- Caceres L [...] Hilgendorf I [...] Rog-Zielinska EA [...]. Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages. Environ Pollut 2024/341:122997
- Fürniss HE, Wülfers EM, Iaconniani P, Ravens U [...] Kohl P, Rog-Zielinska EA*, Peyronnet R*. Disease severity, arrhythmogenesis, and fibrosis are related to longer action potentials in tetralogy of Fallot. Clin Res Cardiol 2023/epub ahead of print
- Kohl P, Greiner, J, Rog-Zielinska EA. Electron microscopy of cardiac 3D nanodynamics: form, function, future. Nat Rev Cardiol 2022/19(9):607-619
- Jünger F [...] Daller CC, Rog-Zielinska EA [...]. 100 Hz ROCS microscopy correlated with fluorescence reveals cellular dynamics on different spatiotemporal scales. Nat Commun 2022/13(1):1758
- Greiner J, Schiatti T, Kaltenbacher W, Dente M, Semenajakin A, Kok T [...] Ravens U, Kohl P, Peyronnet R, Rog-Zielinska EA. Consecutive-Day Ventricular and Atrial Cardiomyocyte Isolations from the Same Heart: Shifting the Cost-Benefit Balance of Cardiac Primary Cell Research. Cells 2022/11(2):233
- Rog-Zielinska EA, Moss R, Kaltenbacher W, Greiner J, Verkade P, Seemann G, Kohl P, Cannell MB. Nano-scale morphology of cardiomyocyte t-tubule/sarcoplasmic reticulum junctions revealed by ultra-rapid high-pressure freezing and electron tomography. J Mol Cell Cardiol 2021/153:86-92
- Rog-Zielinska EA [...] Peyronnet R, Zgierski-Johnston, Greiner J, Madl J [...] Kohl P. Beat-by-beat cardiomyocyte T-tubule deformation drives tubular content exchange. Circ Res 2021/128:203-215
- MacDonald EA, Madl J, Greiner J […] Rog-Zielinska EA*, Quinn TA*. Sinoatrial node structure, mechanics, electrophysiology and the chronotropic response to stretch in rabbit and mouse. Front Physiol 2020/11:908 (*joint authorship)
- Toomer KA […] Kohl P, Rog-Zielinska EA [...] Norris RA. Primary cilia defects causing mitral valve prolapse. Sci Transl Med 2019/11:eaax0290
- Brandenburg S […] Kohl P, Rog-Zielinska EA, Lehnart SE. Junctophilin-2 expression rescues atrial dysfunction through polyadic junctional membrane complex biogenesis. JCI Insight 2019/4:e127116
- Rog-Zielinska EA [...] Zgierski-Johnston CM […] Kohl P. Species differences in the morphology of transverse tubule openings in cardiomyocytes. Europace 2018/20:120-124
- Kong CHT*, Rog-Zielinska EA*, Kohl P, Orchard CH, Cannell MB. Solute movement in the t-tubule system of rabbit and mouse cardiomyocytes. PNAS USA 2018/115:E7073-E7080 (*joint authorship)
- Rog-Zielinska EA, O'Toole ET, Hoenger A, Kohl P. Mitochondrial Deformation During the Cardiac Mechanical Cycle. Anat Rec 2018/302:146-152
- Scardigli M [...] Rog-Zielinska EA, Kohl P [...] Sacconi L. Quantitative assessment of passive electrical properties of the cardiac T-tubular system by FRAP microscopy. PNAS USA 2017/114:5737-5742
- Brandenburg S [...] Rog-Zielinska EA [...] Kohl P [...] Lehnart SE. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest 2016/126:3999-4015
- Quinn TA*, Camelliti P*, Rog-Zielinska EA* [....] Kohl P. Electrotonic coupling of excitable and non-excitable cells in the heart revealed by optogenetics. PNAS USA 2016/113:14852-14857 (*joint authorship)
- Rog-Zielinska EA, Johnston CM [...] Kohl P. Electron tomography of rabbit cardiomyocyte three-dimensional ultrastructure. Prog Biophys Mol Biol 2016/121:77-84
- Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The Living Scar - Cardiac Fibroblasts and the Injured Heart. Trends Mol Med 2016/22:99-114
- Iribe G, Ward CW [...] Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 2009/104:787-795
Team

Eva Rog-Zielinska, PhD
Head of section

Dr. Josef Madl
Senior Scientist
E-Mail: josef.madl@uniklinik-freiburg.de

Leonardo Sacconi, PhD
Senior Scientist

Hannah Kappler

Sofía Orós Rodrigo

Jiaying (April) Fu
E-Mail: jiaying.fu@uniklinik-freiburg.de

Wenzel Kaltenbacher

Wesley Dean Jones