Complement system and CRP

Dr. Oscar Winninger

Johannes Zeller
Modulation of the innate immune system by understanding CRP-complement interaction
Confocal fluorescence microscopy of human neutrophils. Upon stimulation with monomeric CRP neutrophils show enhanced expression of complement 5a receptor 1 (C5aR1). Nucleus - DAPI (blue), anti-C5aR1-FITC (green), 63x oil, scale bar 10 µm
The complement system and C-reactive protein are both part of the innate immune system. While both are indispensable for an effective immune response, both must be kept in narrow tracks and exacerbation is detrimental for the host. In polytrauma these strict regulations fail and patients suffer from an uncontrolled activation of the conplement system. Activation of the complement factor C5 is of particular importance in this disregulated processes:
Pro-inflammatory effects of monomeric CRP are primarily mediated by the Fc-γ receptor family and induces an activation of the complement system cascade when bound to nectrotic or disturbed cell membranes. Thereby, the bioactive conformation of CRP enhances tissue damage as a function of the complement system and via Fc-γ receptors.
This DFG funded study aims to elucidate molecular mechanism of the complement dependent and independent pro-inflammatory effects of CRP. The clinical relevance and feasable therapeutic implications are etablished in "second hit" in vivo models. This study is conducted in cooperation with the Institute of Clinical and Experimental Trauma Immunology, University Hospital Ulm, where a clinical study arm is implemented parallelly.
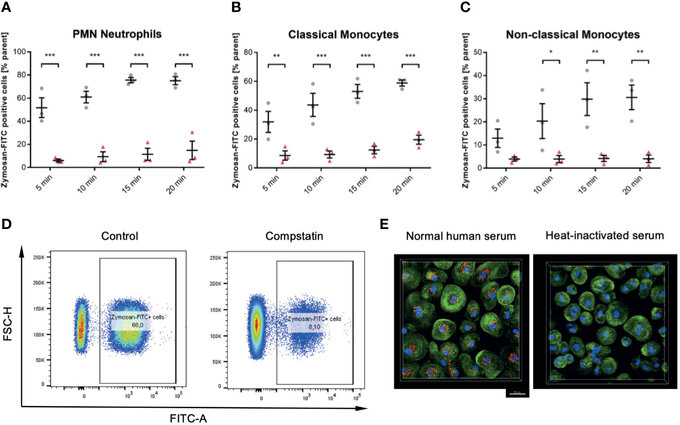
Figure 6 Inhibition of the C3 convertase by Compstatin reduces phagocytosis of zymosan dramatically in whole blood leukocytes. Whole blood treated with 50 µM Compstatin 15 min before the exposure to the phagocytosis targets presented significantly reduced phagocytosis in all assessed subtypes. Compstatin-treated neutrophils showed massively reduced phagocytotic activity at any given time point (A, *** P < 0.001). In classical monocytes (B), the difference to an untreated control for Zymosan-positive cells was the lowest at 5 min time-point (** P < 0.01), with only minor change over time in the Compstatin-treated specimen, resulting in an increase of difference (*** P < 0.001 for 10, 15, and 20 min). Non-classical monocytes (C) showed a similar tendency with the 5 min time-point not significant (P > 0.05), but the following time-points with increasing significant differences to the untreated control (* P < 0.05 for 10 min and ** P < 0.01 for 15 and 20 min). The results are presented as means and S.E.M. and P values were calculated with ANOVA and Tukey post hoc test, n = 3, *, ** and *** P < 0.05, < 0.01 and < 0.001, respectively. (D) shows representative results from phagocytosis by PMNs at the 15 min time-point (E). The overall substantial role in the phagocytosis of zymosan-particle by monocytes was verified by confocal fluorescence microscopy in normal human serum and heat-inactivated serum, respectively. After 45 min of phagocytosis, cells without functioning complement showed significantly less engulfed zymosan (red). Scale bar indicates 20 µm. J Zeller et al., Frontiers in Immunology, Aug 2021
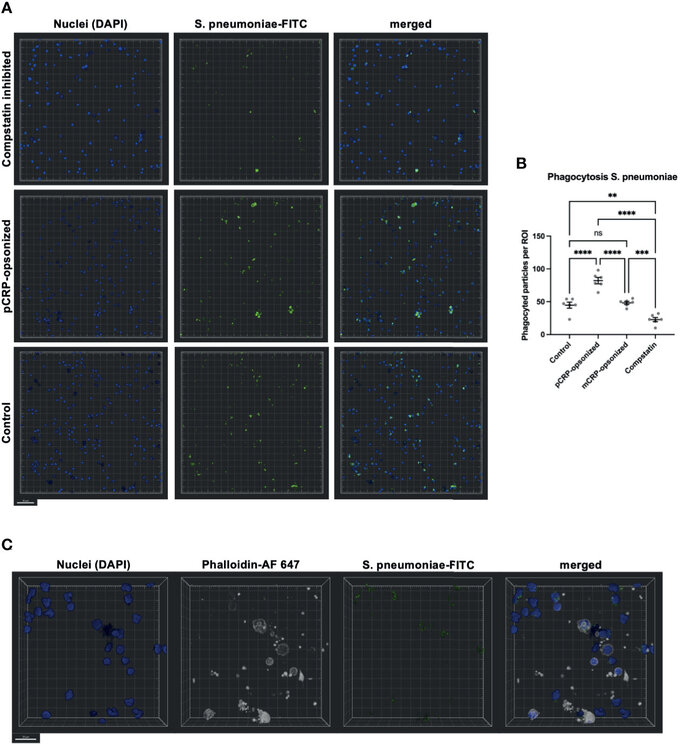
Figure 8 Phagocytosis in whole blood is impeded by inhibition of complement and enhanced by pCRP-opsonization as measured by confocal fluorescence microscopy. Blood was treated as described above. 15 min before the exposure to the phagocytosis targets, the relevant inhibitor was added, and phagocytosis was assessed at 20x magnification. Shown are representative results for pCRP-opsonized S. pneumoniae, Compstatin inhibited cells, and the untreated control group, respectively. Compstatin-treated phagocytic leukocytes (DAPI, blue) showed significantly reduced activity in the phagocytosis of S. pneumoniae-FITC targets (green). When pCRP was added to the targets first, the number of phagocytized particles was significant higher. Scale bar indicates 50 µm (A). However, mCRP added to the targets showed no difference to the unstimulated control. Results are presented as means and S.E.M. and P values were calculated with ANOVA and Tukey post hoc test, n = 6, ns, not significant, ** P < 0.01, *** and **** < 0.001, and < 0.0001 (B). Only FITC-positive targets (green) in direct proximity to the nucleus (blue) and surrounded by F-actin (white) were assumed to be phagocyted particles. Scale bar indicates 20 µm (C). J Zeller et al., Frontiers in Immunology, Aug 2021