Vascular Immunology
Principal Investigator: Prof. Dr. Dennis Wolf
Scientific mission
The Vascular Immunology Laboratory focuses on inflammatory and immune cell mechanisms in cardio-metabolic disease with a particular emphasis on atherosclerosis, adipose tissue inflammation, myocardial infarction, and immune cell trafficking. Atherosclerosis is the most life-threatening pathology worldwide. Its major clinical complications, stroke, myocardial infarction, and heart failure are on the rise in many regions of the world - despite considerable progress in understanding cause, progression, and consequences of atherosclerosis. Atherosclerosis was originally perceived as a lipid- storage disease of the arterial wall but research in the late 80s increasingly established that chronic inflammation in the vasculature critically drives and maintains cardiovascular disease. This inflammatory response involves the presence and accumulation of lymphocytes in the vessel wall that can trigger or inhibit inflammation. It got increasingly clear that lymphocytes and myeloid cells in the vessel wall respond to stimuli and antigens expressed or deposited in the vessel wall, such as low-density lipoprotein (LDL) cholesterol. Vascular immunology is the discipline to describe, understand, and modify the immune response in the vessel wall. Manipulating inflammation and immunity both holds promise for new therapeutic strategies in cardiovascular disease. Ongoing work also suggests that it may be possible to develop antigen-specific immunomodulatory prevention and therapy - a vaccine against atherosclerosis. We are dedicated to contributing to this emerging field and have established a variety of basic and specialized experimental methods, lab techniques, such as immune-cell phenotyping by FACS, time-of-flight mass cytometry (CyTOF), confocal and two-photon imaging, gene expression profiling (RNA-seq.), and various animal methods (intravital microscopy, adoptive transfers, atherosclerosis, adipose tissue inflammation, myocardial infarction, intimal hyper-proliferation, in vivo thrombosis), some of which are carried out in close collaboration with a network of atherosclerosis and immunology labs around the world. Besides, we are also employing various basic lab techniques (molecular biology, protein-biochemistry, and cell culture). Several MD-, Master-, and PhD students were trained in the laboratory since 2009.
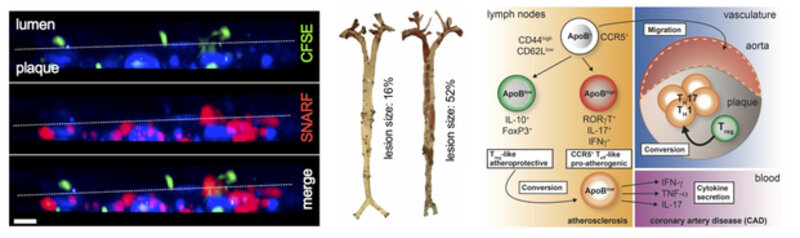
Immune cells are interaction in vessel wall: Two-photon microscopy of an explanted atherosclerotic aorta from an Apoe-knock out mouse that develops atherosclerosis when fed a high-cholesterol diet. In this mouse, antigen-presenting cells (APCs) are labeled with a blue fluorochrome (CD11c-YFP reporter). ApoB-specific T cells (red) are added and their infiltration into the tissue and their interaction with APCs was monitored over 12 hours.

Prof. Dr. Dennis Wolf, MD
Deputy Medical Director, Attending Physician, Principal Investigator
+49-761-270-35460
+49-761-270-34010


Daniela Stallmann
Lab Manager

Dr. rer. nat. Timoteo Marchini
PhD, Deputy Lab Head

Dr. med. Mark Colin Gissler
MD, Post-doc
mark.colin.gissler@uniklinik-freiburg.de

Dr. med. Patrick Siegel
MD, Post-doc

Dr. med. Istvan Bojti
MD, Post-doc

Hauke Horstmann
MD, Post-doc

Lucia Sol Mitre
MD, MSc (IMBS), PhD-student (SGBM)

Sheu-Tijani Olawale Abogunloko
MD, MSc (IMBS), PhD-student (SGBM)

Juana Dominguez
MD, MSc (IMBS), PhD-student (SGBM)

Xin Gu
MD Student

Timothy Bon-Nawul Mwinyella
BSc, Technical assistant

Sophie Hansen
MD student, MOTI-VATE scholar

Xiaowei Li
MD student

Ana-Sophia Burkard
MD-student

Simon Heitlinger
MD-student

Katharina Wißkirchen
MD-student

Cynthia Morguet
MD-student

Noah Jung
MD-student (MOTIVATE-scholar)

Antonia Ziegler
MD-Student

Elena Plank
MD student

Rebecca Janiak
MD Student
Alumni
- Anton Alexander (Msc-thesis "Comprehensive Characterization of Immune Cell Diversity in Murine Aortic Layers by Single Cell RNA Sequencing")
- Son Guk Cho Kim, Technical Assistant
- Aitana de la Cruz Tabernero, Technical Assistant
- Dr. rer. nat. Lourdes Caceres ("Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages")
- Fabienne Ehret (MSc-thesis: Detection and characterization of tissue resident T cells in aortic intimal, medial, and adventitial layers of healthy mice)
- Mona Reuvers, Lab manager
- Dr. med. Teresa Gerhardt, MD (MD-thesis: “Loss of protective autoimmunity in atherosclerosis“)
- Dr. rer. nat. Nathaly Anto-Michel, PhD, MSc (MSc-thesis: “Characterization of a novel antibody targeting the CD40L/Mac-1 interaction in vascular disease”, PhD-thesis: “The Role of Tumor Necrosis Receptor associated Factor (TRAF) -1 in cardio-metabolic disease“)
- Lucia Sol Mitre (MSc-thesis: “Characterization of the immune cell repertoire in Myocardial Infarction and Heart Failure“)
- Dr. med. Hermann Blankenbach (MD-thesis: “Generierung und Charakterisierung eines neuen Liganden- und Aktivitäts-spezifischen Antiköpers zur selektiven Hemmung der CD40L/Mac-1 Interaktion“)
- Dr. med. Ansgar Wiedemann (MD-thesis: „Mechanismen der CD40L/Mac-1 vermittelten Inflammation“)
- Dr. med. Felix Jehle (MD-thesis: „Die Rolle von CD40 Ligand und seinem Rezeptor CD40 in der Pathogenese des Metabolischen Syndroms“)
- Dr. med. Eva Nora Bukosza (MD-thesis: “Contribution of the CD40L/Mac-1 pathway to the metabolic syndrome”)
- Maria Reina Campos (BSc-thesis: “Development of an ELISPOT assay for the detection of the ApoB100-specific CD4+ T cell population in patients with cardiovascular disease")
- Xia Sheng (MD-student)
- Guido Pisani (MSc-thesis: “Detection and Characterization of Resident Memory T Cells in the Vasculature of Healthy Mice”)
- Abed Al Hadi El Rabih (MSc-thesis: “The role of endothelial CD40 ligand in myocardial infarction and cardiac remodelling in mice”)
- Philipp Scherrer (MD-thesis: “Die Rolle von endothelialem CD40L in vaskulärer Inflammation“)
- Sara Malchow (BSc-thesis: “Developing and Applying an ELISA for IgG-ApoB-Autoantibodies”)
We are currently accepting applications for MD-, Master-, PhD students, and for Postdoctoral Fellows at dennis.wolf@uniklinik-freiburg.de. MD students can choose between an experimental thesis work in the laboratory or a clinical-experimental thesis with patient samples. Students will be trained in all required methods, animal care, as well as in project design, data analysis, and presentation. They will be offered with the chance to present data on scientific conferences and will be supported with applications for grants or stipends. Full-time work for one year in the laboratory is required.
Please provide a C.V. and a summary of your previous scientific work and/or your scientific interest.
CORE PROJECT 1:
Defining the adaptive immune response to develop a vaccine against atherosclerosis
Most people worldwide die of cardiovascular disease, such as of heart attack and stroke. Often, the underlying cause is atherosclerosis, a disease that fuels the build-up of vessel-narrowing plaques in arteries that are vital because they supply the heart and the brain. During early disease, a subgroup of white blood cells called T cells infiltrate the plaque and drive its growth. Usually, these immune cells protect from infection, but in atherosclerosis some T cells attack the body itself. This is why atherosclerosis is now understood as auto-immune disease. They get attracted by the protein ApoB-100, which is a part of LDL-cholesterol in the plaque. However, little is known about the function of these self-reactive T cells and how to dampen their harmful action. Using innovative tools, such as 2-photon microscopy of explanted atherosclerotic plaques (see above, left), combined with classical atherosclerosis tools, such as quantification of atherosclerotic lesions in mice (middle), and MHC-multimers to track single auto-reactive T cells we are asking some intriguing questions: What is the frequency and function of autoreactive T cells? Which proteins and genes do they express? When transferred into another animal, how do these autoreactive T cells change atherosclerosis in the recipient? We are also trying to track auto-reactive T cells in humans with coronary artery disease. Deciphering how the immune system and the T cell response work in atherosclerosis will not only boost our understanding of auto-immunity in atherosclerosis but it will also build the basis to define new therapies. Our long-term goal is to define the properties of a vaccine against ApoB-100 to modify the immune system in a way that protective autoreactive T cells are boosted. Such therapy would represent the first causal preventive therapy against the atherosclerosis-related immune response in future.
Suggested reading:
- Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4+ T-Regulatory Cells
link: https://pubmed.ncbi.nlm.nih.gov/32703007/ - Immunity and Inflammation in Atherosclerosis.
link: https://pubmed.ncbi.nlm.nih.gov/30653442/ - Regulatory CD4+ T Cells Recognize MHC-II-Restricted Peptide Epitopes of Apolipoprotein B
link: https://www.ncbi.nlm.nih.gov/pubmed/29588316
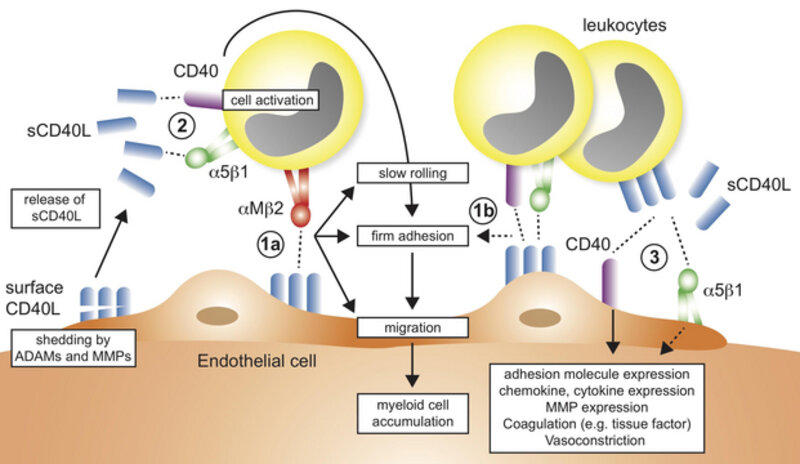
CORE PROJECT 2:
Therapeutic targeting of integrins in inflammation
The recruitment of leukocytes to inflamed tissue is a necessary step to resolve inflammation and infection in tissues. However, this initially protective attempt is often dysregulated and initiates a vicious circle that attracts more leukocytes, drives inflammatory cytokine secretion, and eventually causes tissue damage. One solution to this problem is to block leukocyte recruitment by inhibiting the molecules on leukocytes that are required for leukocytes to attach to the endothelium and to transmigrate into the tissue. Yes, this may be problematic, because adhesion molecules, e.g. leukocyte integrins, are simultaneously needed for many beneficial functions, such as phagocytosis, bacterial clearance and cell-cell interactions. We found that the interaction of one leukocyte integrin, Mac-1, with the immune molecule CD40L specifically regulates harmful cell recruitment by binding to endothelial CD40L and serving as adhesion receptor (see schematic above) without interfering with Mac-1’s protective functions in host defense, thrombosis, and wound healing. We designed an antibody and a peptide to specifically neutralize this receptor-ligand interaction, while leaving other potentially beneficial functions of the leukocyte integrin untouched. Our work documents a novel therapeutic strategy that protects from atherosclerosis and sepsis without relevant side effects observed in conventional treatment strategies. Two international patents arose from this work, which now serve as basis to ultimately develop a novel leukocyte recruitment blocker for human disease. We are using with a variety of protein assays to monitor the interaction of proteins and inhibitors and testing these directly in vivo in intravital microscopy and several inflammatory models, such as in experimental sepsis or peritonitis. Currently, we are also testing whether the specific inhibitor of the CD40L/Mac-1 interaction prevents adverse cardiac remodeling after myocardial infarction.
Suggested reading:
- A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense
ink: https://www.ncbi.nlm.nih.gov/pubmed/29410422 - CD40L and Its Receptors in Atherothrombosis-An Update
link: https://www.ncbi.nlm.nih.gov/pubmed/28676852 - Binding of CD40L to Mac-1's I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis - but does not affect immunity and thrombosis in mice
link: https://www.ncbi.nlm.nih.gov/pubmed/21998326
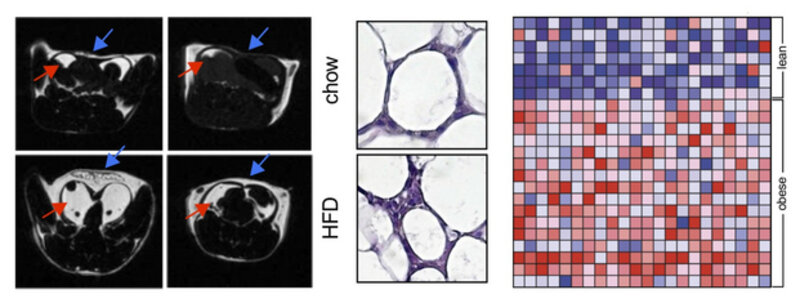
CORE PROJECT 3:
Finding inflammatory signaling pathways in cardio-metabolic disease
Atherosclerosis, its acute complication myocardial infarction, as well as other cardiovascular disease is initiated and maintained by cardiovascular risk factors, such as obesity, insulin resistance, hypertension, hyperlipidemia, often referred to as the Metabolic Syndrome. It is now well established that some components of the Metabolic Syndrome, such as obesity and insulin resistance, drive an inflammatory response in visceral adipose tissue. It is, however, not known to which extend adipose tissue inflammation contributes to atherosclerosis and whether mutual inflammatory pathways exist that are druggable to define therapies that target both: risk factors and vascular disease simultaneously. We have found that CD40L, a costimulatory molecule initially described on activated T cells, is expressed on adipocytes, particular in obese, inflamed adipose tissue. Functionally, mice with a genetic deficiency of CD40L are protected from inflammation, while a deficiency of its classical interaction partner CD40 induces a hyper-inflammatory phenotype in adipose tissue and aggravated dysmetabolism in mice. A deficiency of the signaling adaptor TRAF-1 that is functioning downstream of CD40- and TNF-signaling resembles this hyper inflammatory phenotype, but unexpectedly protects from dysmetabolism. These findings document a novel role of CD40 as co-inhibitory molecule on T cells and suggest that activating CD40 antibodies – as currently tested in cancer trials – can protect from cardio- metabolic disease. Targeting inflammation of visceral adipose tissue alone may, however, not suffice to improve metabolic risk factors. We have developed and used a broad repertoire of methods and techniques to study metabolism in mice, including monitoring fat depositions by MRI (see Figure above, left), histo-morphology of adipocytes (middle), metabolic chambers, and genetic tools to screen for the expression of inflammatory pathways in human fat tissue by gene enrichment analysis (right).
Suggested reading:
- Inflammatory Pathways Regulated by Tumor Necrosis Receptor-Associated Factor 1 Protect From Metabolic Consequences in Diet-Induced Obesity
link: https://www.ncbi.nlm.nih.gov/pubmed/29358227 - Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice
link: https://www.ncbi.nlm.nih.gov/pubmed/24664276 - CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies
link: https://www.ncbi.nlm.nih.gov/pubmed/22412980
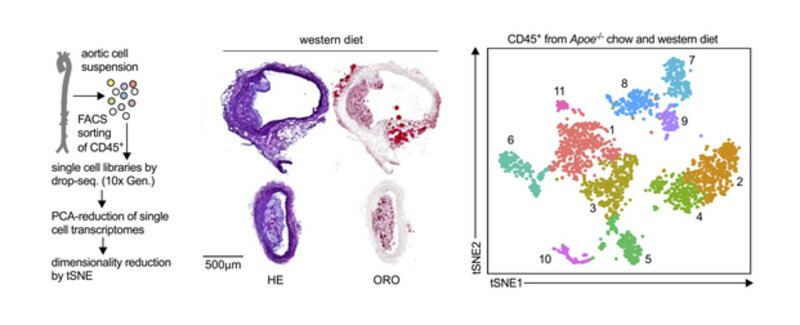
CORE PROJECT 4:
Depicting leukocyte heterogeneity in atherosclerosis
The development and progression of atherosclerosis is predominately driven by leukocyte infiltration and accumulation in atherosclerotic plaques. Experimentally, atherosclerosis can be prevented and treated by modulating the influx and the function of pro-inflammatory leukocytes. The exact contribution of different leukocyte lineages, their phenotypes, and the existence of atherosclerosis-specific leukocyte subsets, however, is largely unknown. Historical protocols to digest and phenotype leukocytes in atherosclerotic mouse aortas are based on classical flow cytometry and have been applied since 2006 to study leukocyte populations in atherosclerosis and other vascular disease models by many labs around the world. Since then, it became increasingly clear that not all leukocytes found in the atherosclerotic aorta are equal; in fact, many principal aortic leukocyte lineages, such as B- or T-cells, encompass both protective and pathogenic sub-populations. This diversity has great implications on understanding the definitive biology and to establish novel cell-based therapeutic strategies. Our inspiration came from recent observations that high-parameter phenotyping in other disease models has the potential to detect novel sub-populations with unexpected combinations of surface markers. These cells may therefore reflect disease-specific leukocytes and identify subsets beyond what flow cytometry and immunohistochemistry can do. We are using a combination of cell sorting of aortic leukocytes in atherosclerotic mouse aortas (see above, middle and left) and single cell RNA-sequencing. These new approaches are less biased, i.e., make fewer assumptions based on pre-conceived notion. Thus, they are effective, efficient and powerful screening tools. Currently, we are aiming to define leukocyte heterogeneity in human atherosclerotic plaques and to test whether particular leukocyte sub-populations in peripheral blood of humans correlate with cardiovascular disease.
Suggested reading:
- Heterogeneity of T Cells in Atherosclerosis Defined by Single-Cell RNA-Sequencing and Cytometry by Time of Flight.
link: https://pubmed.ncbi.nlm.nih.gov/33267666/ - Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas.
link: https://pubmed.ncbi.nlm.nih.gov/32673538/ - Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry
link: https://www.ncbi.nlm.nih.gov/pubmed/29545366
CORE PROJECT 5:
The role of air pollution in cardiovascular disease
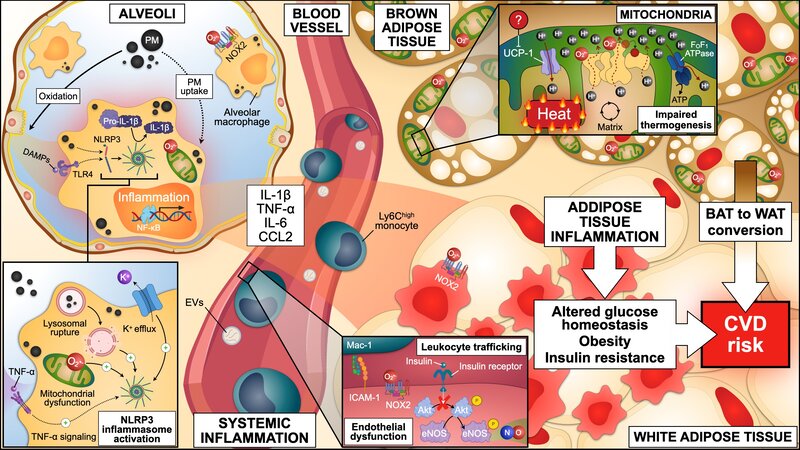
Air pollution stands as the single largest environmental health risk factor, responsible for approximately 7 million premature deaths worldwide each year. Among the different components that are present in polluted air, airborne fine particulate matter below 2.5 μm in diameter (Feinstaub, PM2.5) has been identified as the primary hazardous constituent. PM2.5 mainly originates from fossil fuel combustion during power generation, industrial processes, and transportation. While the lungs are in first contact with PM2.5 after inhalation, the main complications of breathing polluted air manifest as cardiovascular diseases, including myocardial infarction and stroke. PM2.5 uptake by lung tissue resident alveolar macrophages triggers a local and systemic inflammatory response, which alters homeostasis at secondary locations, such as the blood vessels, the heart, the brain, and fat depots. Consequently, exposure to PM2.5 aggravates myocardial infarction, impairs cardiac lesion remodelling, and favours the progression to heart failure. In the last decade, it has been increasingly recognized that PM2.5 exposure accelerates the development of risk factors for cardiovascular disease (refer to the figure below), such as hypertension and diabetes. However, mechanistic links between PM2.5-driven inflammation and metabolic derangements remain unclear. We are combining a variety of in vivo, ex vivo, and in vitro experimental models of exposure to PM2.5 to unravel the contribution of air pollution to cardiovascular disease onset and progression, and to evaluate inhibitory approaches to deal with this high-impact public health issue.
Suggested reading:
- Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages.
link: https://pubmed.ncbi.nlm.nih.gov/38000727/ - Redox and inflammatory mechanisms linking air pollution particulate matter with cardiometabolic derangements.
link: https://pubmed.ncbi.nlm.nih.gov/37852544/ - Pathogenic role of air pollution particulate matter in cardiometabolic disease: evidence from mice and humans.
link: https://pubmed.ncbi.nlm.nih.gov/32403947/ - Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages.
link: https://pubmed.ncbi.nlm.nih.gov/27240856/
1. Longitudinal Assessment of Subclinical Arterial Inflammation in Patients Receiving Immune Checkpoint Inhibitors by Sequential [(18)F]FDG PET Scans. Bacmeister L, Hempfling N, Maier A, Weber S, Buellesbach A, Heidenreich A, Bojti I, Gissler MC, Hilgendorf I, von Zur Muehlen C, Westermann D, Meyer PT, Goetz C, Wolf D. Circ Cardiovasc Imaging. 2025 Feb;18(2):e016851.
2. Cross-species single-cell RNA sequencing reveals divergent phenotypes and activation states of adaptive immunity in human carotid and experimental murine atherosclerosis. Horstmann H, Michel NA, Sheng X, Hansen S, Lindau A, Pfeil K, Fernández MC, Marchini T, Winkels H, Mitre LS, Abogunloko T, Li X, Mwinyella TB, Gissler MC, Bugger H, Heidt T, Buscher K, Hilgendorf I, Stachon P, Piepenburg S, Verheyen N, Rathner T, Gerhardt T, Siegel PM, Oswald WK, Cohnert T, Zernecke A, Madl J, Kohl P, Foks AC, von Zur Muehlen C, Westermann D, Zirlik A, Wolf D. Cardiovasc Res. 2024 Nov 25;120(14):1713-1726.
3. Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4+ T-Regulatory Cells. Wolf D, Gerhardt T, Winkels H, Michel NA, Pramod AB, Ghosheh Y, Brunel S, Buscher K, Miller J, McArdle S, Baas L, Kobiyama K, Vassallo M, Ehinger E, Dileepan T, Ali A, Schell M, Mikulski Z, Sidler D, Kimura T, Sheng X, Horstmann H, Hansen S, Mitre LS, Stachon P, Hilgendorf I, Gaddis DE, Hedrick C, Benedict CA, Peters B, Zirlik A, Sette A, Ley K.
Circulation. 2020 Sep 29;142(13):1279-1293.
4. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Wolf D, Anto-Michel N, Blankenbach H, Wiedemann A, Buscher K, Hohmann JD, Lim B, Bäuml M, Marki A, Mauler M, Duerschmied D, Fan Z, Winkels H, Sidler D, Diehl P, Zajonc DM, Hilgendorf I, Stachon P, Marchini T, Willecke F, Schell M, Sommer B, von Zur Muhlen C, Reinöhl J, Gerhardt T, Plow EF, Yakubenko V, Libby P, Bode C, Ley K, Peter K, Zirlik A.
Nature communications, 2018; 9(1):52
5. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA- Sequencing and Mass Cytometry. Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K, Wolf D.
Circulation Research. 2018; 122(12):1675-1688
Full list of publications:
August 2025
- PhD student Tijani Abogunloko receives a Travel Award for the annual meeting of the European Society of Cardiology (EAS)
May 2025
- PhD student Tijani Abogunloko receives a Travel Award for the annual meeting of the European Atherosclerosis Society (EAS)
March 2025
- MD-student Antonia Ziegeler receives a scholarship from the German Society of Internal Medicine (DGIM)
February 2025
- Hauke Horstmann is awarded with the prestigious Werner-Hauss-Award of the German Society of Arteriosclerosis Research during the Vascular Medicine and Atherosclerosis Congress (VMAC)
- Timoteo Marchini receives the Poster Award at the Vascular Medicine and Atherosclerosis Congress (VMAC)
July 2024
- Hauke Horstmann is awarded with the Edith-von-Kaulla Preis by the Faculty of Medicine Freiburg
March 2024
- Dr. Timoteo Marchini is awarded with the ECR Fellowship of the Society for Free Radical Research-Europe (SFRR-E)
January 2024
- MD student Ana-Sophia Burkard is awarded with the Eberhard-Betz Prize of the German Society of Atherosclerosis-Research (DGAF)
July 2023
- MD student Noah Jung joins the MOTIVATE program of the University of Freiburg in 2021
April 2023
- Hauke Horstmann is awarded with the Young Investigator Award of the German Society for Internal Medicine (DGIM).
February 2023
- Dr. Patrick Siegel is funded by the clinician scientist fellowship from SFB1425.
January 2023
- Dr. Timoteo Marchini is funded by the Research Commission of the Faculty of Medicine of the University of Freiburg
October 2022:
- Dr. Mark Colin Gissler receives the Ludolf Krehl Preis of the Südwestdeutsche Gesellschaft für Innere Medizin
- MD student Sophie Hansen is awarded with the Otto-Hess poster prize by the German Cardiac Society (DGK)
- MD student Julius Wissemann receives the Otto-Hess-Scholarship of the German Cardiac Society.
September 2022
- MD student Hauke Horstmann is awarded with the Poster Prize at the GEMSEQ expert meeting in Graz.
August 2022
- Dr. Mark Colin Gissler is awarded with the Edith von Kaulla Research Award of the University of Freiburg
June 2022
- Dr. Timoteo Marchini receives "The Future of Redox Biology Award" from the Society for Free Radical Research International
May 2022
- Dr. Mark Colin Gissler is awarded with the Young Investigator Fellowship of the European Atherosclerosis Society (EAS)
April 2022
- MD student Hauke Horstmann receives the Travel Award of the German Cardiac Society (DGK)
March 2022
- Dr. Mark Colin Gissler is awarded with the Poster Award at this year’s meeting of the German Society of Atherosclerosis-Research (DGAF).
- Dr. Mark Colin Gissler receives the Eberhard-Betz-Price of the German Atherosclerosis Society (DGAF)
- MD student Sophie Hansen receives the Kaltenbach-Scholarship of the German Heart Foundation.
January 2022
- Dr. Mark Colin Gissler is selected into the DFG-funded “IMM-PACT” clinician scientist program of the University of Freiburg
August 2021
- MD student Hauke Horstmann receives the best abstract award by the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology (ESC)
July 2021
- Lourdes Caceres receives a scholarship from the German Academic Exchange Service (DAAD)
June 2021
- Dr. Timoteo Marchini receives the Young Investigator Award of the Society for Free Radical Research Europe
- MD student Katharina Wißkirchen will join the MOTIVATE program of the University of Freiburg in 2021
- Dennis Wolf receives 1 Million Euro funding for an endowed professorship for “Cardiovascular Systems Immunology” from the Else-Kröner-Fresenius-Stiftung.
April 2021
- Dennis Wolf elected as deputy speaker of the working Group “Heart and Diabetes” of the German Society for Cardiology (AG23, DGK)
- PostDoc and MD Colin Gissler is awarded with the Young Investigator Award of the German Society for Internal Medicine (DGIM).
March 2021
- Dr. Timoteo Marchini receives the Young Investigator Award of the Society for Free Radical Research International (SFRR-I)
- MD student Hauke Horstmann is awarded with the Gotthard-Schettler Young Investigator Award of the German Society of Atheroslerosis-Research (DGAF)
December 2021
- MD student Sophie Hansen receives the Otto-Hess Scholarship of the German Society of Cardiology (DGK)
November 2021
- Dennis Wolf is awarded with the Edith von Kaulla Forschungspreis of the Medical Faculty of the University of Freiburg.
June 2020
- The Vascular Immunology Laboratory joins the newly established collaborative research center (Sonderforschungsbereich) SFB1425 (“Heterocellular Nature of Cardiac Lesions: Identities, Interactions, Implications”)
May 2020
- MD student Hauke Horstmann receives the Kaltenbach-Scholarship the German Heart Foundation
March 2020
- Timoteo Marchini awarded with the Gotthard-Schettler Young Investigator Award of the German Society of Atheroslerosis-Research (DGAF)
November 2019
- Dennis Wolf is appointed as principal investigator in the Spemann Graduate School of Biology and Medicine (SGBM)
- MD student Philipp Scherrer receives the Otto-Hess Scholarship of the German Society of Cardiology (DGK)
October 2019
- Dennis Wolf receives a prestigious starting grant with 1.5 Million Euro funding from the European Research Council (ERC)
- Dennis Wolf is awarded with the Hans-Jürgen-Bretschneider Poster Award of the German Society of Cardiology (DGK)
September 2019
- MD student Mark Colin Gissler is awarded for the Best Moderated Poster in “Vascular Signaling” during the congress of the European Society of Cardiology in Paris
- MD student Mark Colin Gissler receives the Basic Science Travel Award of the European Society of Cardiology
July 2019
- MD student Hauke Horstmann receives the Poster Award at the Summer School Meeting of the University Heart Centre Freiburg-Bad Kronzingen (Cardiology and Angiology I)
- MOTIVATE class of 2019, MD student Sophie Hansen will join the MOTIVATE program in 2019
- MSc student Tijani Olawale receives the Academic Excellence Scholarship of the German Academic Exchange Service (DAAD)
May 2019
- Dennis Wolf is elected as member of the Advisory Board of the German Society of Atherosclerosis Research (DGAF)
- Dennis Wolf receives the W.H. Hauss Award of the German Society of Atherosclerosis Research (DGAF)
- Dennis Wolf is awarded as “Paul Dudley White International Scholar” at the Vascular Discovery Meeting of the American Heart Association
- MD student Mark Colin Gissler is nominated as finalist of the Young Investigator Award of the German Society of Atherosclerosis Research (DGAF) competition
- Dennis Wolf receives the Berta-Ottenstein Advanced Clinician Scientist Fellowship
2018
- MD student Philipp Scherrer receives the Kaltenbach Fellowship from the German Heart Foundation, November 2018
- MOTIVATE class of 2018, MD student Hauke Horstmann will join the MOTIVATE program in 2019
- Leukocyte Heterogeneity in Atherosclerosis, Cover Page Circulation Research, 8th June 2018
- First Giant Steps Toward a Cell Atlas of Atherosclerosis, Editorial on our article, Circulation Reseach, 8th June 2018
- Featured Research Highlight in Nature Reviews Cardiology,10th April 2018
- Otto-Hess Promotionspreis, MD student Teresa Gerhardt awarded by the German Society of Cardiology.
Press features
- Feature of our work in the EU Research Magazine Horizon
- Meet the First Authors, former Postdoc Nathaly Anto Michel featured by Circulation Research, 2nd March 2018
- “Immun gegen den Herzinfarkt?”, Frankfurter Allgemeine Wochenzeitung, 11th July 2016
- Young Investigator Spotlight, Dennis Wolf featured by Circulation, January 31st 2012
Kontakt

Prof. Dr. Dennis Wolf
Freiburg: +49-761-270-35460
Bad Krozingen: +49-7633-402-4277
Labor/Lab: +49-761-270-70371